The vaccine also was 85 effective at preventing severe disease with the COVID-19 virus at least 28 days after vaccination. Vaccination against measles protects against infection for life in 96 of people.

Can The Covid Vaccine Protect Me Against Virus Variants The New York Times
COVID-19 vaccines also help keep you from getting seriously ill even if you do.

How long will covid vaccine protect me for. COVID-19 vaccines are safe and effective. How long will a vaccines protection against Covid-19 last. How long will vaccines continue to protect against COVID-19 30 July 2021.
A recent study from the University of Washington found that 30 of Covid patients. Learn more about how COVID-19 vaccines work. They can keep you from getting and spreading the virus that causes COVID-19.
The Joint Committee on Vaccination and Immunisation is expected to rule soon on a booster plan for the UK. If you are not vaccinated find a vaccine. COVID 19-vaccines are effective.
Regardless of which vaccine you get you wont reach full protection until two weeks after your second or final dose. A 2004 study of individuals who were vaccinated against measles as children found strong T-cell immunity more than 30 years later. This vaccine is for people age 18 and older.
That means its possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and still get sick. It requires one injection. After receiving a COVID-19 vaccine it takes weeks for your immunity to build.
PDF 513KB 9 pages. But the takeaway for now. According to the CDC full protection occurs two weeks after the second dose of the Pfizer-BioNTech or Moderna COVID-19 vaccines or two weeks after the single-dose Johnson Johnson vaccine.
1 1 Covid vaccine protection waning in those first jabbed study suggests. You may have side effects after vaccination but these are normal. It typically takes two weeks after you are fully vaccinated for the body to build protection immunity against the virus that causes COVID-19.
Protection from Pfizers two-dose vaccine remains above 91 even at six months according to the company. August 26 2021 By In COVID. One is that the three coronavirus vaccines authorized for use in the United States provide a high degree of protection for at least three months based on clinical trials that began as early as last.
Mandates and shockingly many of the government-sponsored vaccine ads go against FTC law regarding deceptive advertising. A US study of essential workers found that a single dose of Pfizer of Modernas COVID-19 vaccines were 80 effective against all coronavirus infections from 14 days. You can see the actual law in this letter sent to all universities currently trying to mandate the COVID shot.
Most of the shot. How long does it take for the COVID-19 vaccine to work. This is undeniably good news but it does not account for long-term outcomes from asymptomatic or mild infections.
We dont know how long T-cell immunity persists after COVID-19 vaccination or infection but theres evidence that T cells last for quite a while. COVID-19 vaccines are effective. Thats about how long it takes your immune system to mount an antibody response to the vaccine.
Should We Have Waited Longer Between COVID-19 Vaccine Doses. It typically takes a few weeks for the body to build immunity protection against the virus that causes COVID-19 after vaccination. Heres what experts have learned about how the time between the Pfizer and Moderna shots affects overall protectionHeres what experts have learned about how the time between the Pfizer and Moderna shots affects overall protection.
A recent study in The Lancet looked at more than 23000 vaccinated healthcare workers in the United Kingdom from December to February and found the Pfizer-BioNTech vaccine was at least. In clinical trials this vaccine was 66 effective in preventing the COVID-19 virus with symptoms as of 14 days after vaccination. Learn more about how federal partners are ensuring COVID-19 vaccines work.
Researchers tracked the symptoms of 66 previously hospitalized long-COVID patients for more than eight months 44 of whom received a vaccine earlier this year. Vaccine mandates for experimental COVID shots are against the law in the US. Overall theres still much to learn before we known for certain how long the COVID-19 vaccines will last says Dr.
Marks says that the vaccines protection is generally achieved somewhere between seven to 14 days after the second dose. All vaccines work this way. Learn more about the different COVID-19 vaccines.
This is a major issue that is now being studied carefully by scientists though they warn it may take some time before they can. In the meantime there are a few things we do know.
Learn more about US. As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States.
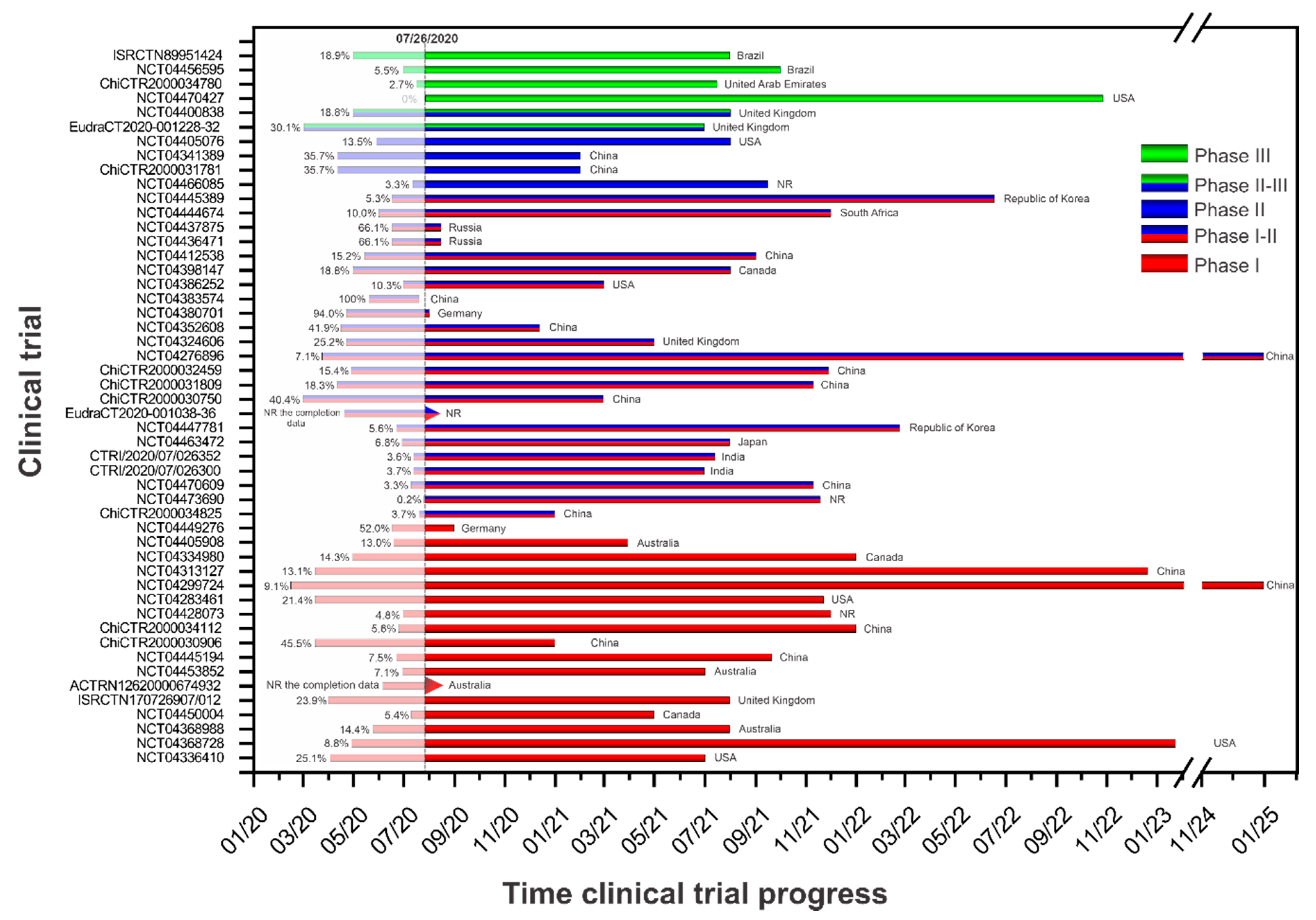
Vaccines Free Full Text Current Clinical Trials Protocols And The Global Effort For Immunization Against Sars Cov 2
Based on data reported by the manufacturer for PfzierBioNTech vaccine BNT162b2 this critical appraisal shows.

Which covid vaccines are in clinical trials. What the trial is looking at. Clinical trials for a COVID-19 vaccine are designed to assess the safety and efficacy of the vaccine. A new clinical trial will investigate whether a third COVID-19 vaccine dose gives a stronger immune response for people with weakened immune systems.
LNB News 25082021 31. Clinical trials for COVID-19 vaccines differed along a surprising number of dimensions based on manufacturer choices. Among them 63 vaccines have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials.
Clinical trials for other COVID-19 vaccines are ongoing including those by Janssen here Novavax here and Moderna here. Indias first homegrown mRNA-based COVID-19 vaccine being developed by Gennova Biopharmaceuticals has been approved for further clinical trials today after. What were the key differences in how clinical trials were run for COVID-19 vaccines.
Up to now six COVID-19 vaccines including two mRNA vaccines two inactivated vaccines and two viral-vectored vaccines have been authorized for emergency use or conditional licensed in some countries or regions based on their efficacy data in phase 3 trials. 2 for clinical trials for medical devices and drugs related to COVID-19. The Pfizer vaccine Comirnaty has been assessed in global studies across three phases.
University of New Mexico School of Medicine researchers have begun enrolling children in a clinical trial to test the safety and efficacy of the Moderna COVID-19 vaccine. 95 CI 900 to 976. This listing represents the initial authorization of the trial but does.
The tables below list the COVID-19-related clinical trials which have been authorized by Health Canada through the Food and Drug Regulations or the Interim order No. March 24 2021 March 4 2022 primary completion date. Phase one and two assessed the safety and immunogenicity the immune response after each dose of different dose levels of the vaccine in a small population.
List of COVID-19 treatment trials. The OCTAVE DUO study will offer people who are immunosuppressed or immunocompromised a third Pfizer Moderna or Novavax vaccine to determine whether this will give a stronger immune response than. NVX-CoV2373 is a protein-based vaccine engineered from the genetic sequence of SARS-CoV-2 the virus that causes Covid-19.
Researchers are analyzing the safety tolerability and immune responses of the COVID-19 vaccine in healthy children under the age of 12 years. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in PfzierBioNTech and Moderna COVID-19 mRNA vaccine clinical trials. JanssenJohnson Johnson COVID-19 vaccine.
MRNA-based vaccines got global focus following the huge success of Pfizer BioNTech and Fosun Pharmas Covid. 89 rows On this page. List of COVID-19 vaccine trials.
COVID-19 vaccine clinical trials including vaccines in earlier stages of development by visiting clinicaltrialsgov. NVX-CoV2373 clinical trials have enrolled more than 30000 volunteers around the globe. The number of doses the spacing between multiple doses the amount of vaccine per dose the patients studied and the endpoints tested.
Absolute risk reduction 07. The Department of Health and Social Care DHSC has announced a new clinical trial to assess whether a third dose of coronavirus COVID-19 vaccines is needed for some clinically vulnerable people. Gennova plans to use the DBT-ICMR clinical trial network sites for this study.
Overview of Twelve Vaccines Targeting COVID-19 in Phase III Clinical Trials Within ten months time twelve vaccine candidates had been officially registered into Phase III clinical trials httpswwwwhointpublicationsmitemdraft-landscape-of-covid-19-candidate-vaccinesaccessed on 9. The vaccine also was 85 effective at preventing severe disease with the COVID-19 virus at least 28 days after vaccination. Clinical trial phase.
In clinical trials this vaccine was 66 effective in preventing the COVID-19 virus with symptoms as of 14 days after vaccination. Novavax COVID-19 vaccine. Pfizer-BioNTech COVID-19 Vaccine is still being studied in clinical trials.
A new clinical trial to determine whether a third dose of vaccine will improve the immune response for people who have weakened immune systems is launching in the UK. Specifically the trial will look at whether a third vaccination dose produces a stronger immune response than two doses in. The FDA also confirms in their fact sheet that the Pfizer jab alongside all other Covid jabs is still in clinical trials Serious and unexpected side effects may occur.
95 CI 059 to 083. Relative risk reduction 951.
The drug made from a combination of two antibodies was initially developed as a treatment for those who had already been exposed to the disease. AstraZeneca hails trial results for COVID treatment Credit.

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet
UnsplashCC0 Public Domain Drug firm AstraZeneca on Friday announced positive results from a trial of a treatment for COVID-19.
Astrazeneca covid 19 vaccine trial results. AstraZeneca has updated the efficacy result of its coronavirus vaccine trial in the US after health officials insisted they wanted to include the latest information. AZD7442 a combination of two long-acting antibodies reduced the risk of developing symptomatic Covid-19 by 77 compared to placebo the. AP PhotoJuan Karita The Russian Direct Investment Fund RDIF has announced the results of a small clinical study that was conducted in the Republic of Azerbaijan.
AstraZeneca said its COVID-19 vaccine was 76 effective. Sciences COVID-19 reporting is supported by the Heising-Simons Foundation. The drug made from a combination of two antibodies was initially developed as a treatment for those who had already been exposed to the disease.
The drug made from a combination of two antibodies was initially developed as a treatment for those. The drug was made from a combination of two antibodies which was initially developed as a treatment for those who had already been exposed to the virus. Trial results suggesting the AstraZeneca-Oxford vaccine might only offer minimal protection against mild and moderate doses of the Covid-19.
The long-awaited results from a new trial of AstraZenecas COVID-19 vaccine hold some good news for the company which. AstraZeneca said its COVID-19 vaccine was 76 effective at preventing symptomatic illness in a new analysis of its major US trial -. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use.
As per MedCity News in addition to the clinical trial data in COVID-19 prevention the company said that preliminary lab test results from Oxford University and Columbia University found that the drug neutralizes variants of SARS-CoV-2 including the Delta strainIn turn AstraZeneca said it will submit full results from the PROVEN clinical trial for publication in a peer-reviewed medical. The results from a trial that looked at how AstraZeneca and Sputnik Light doses reacted when combined. AstraZeneca PLC said late-stage trials for the Covid-19 vaccine it is developing with the University of Oxford are on track to produce results later this year with a potential rollout soon.
As a result Covid-19 was removed from the list of HCIDs. First peer-reviewed results of phase 3 human trials of Oxford coronavirus vaccine demonstrate efficacy CoronavirusCOVID-19 vaccine Our vaccine work is progressing quickly. And this strategy has succeeded in creating higher immunity towards the virus.
LONDON Aug 20 Drug firm AstraZeneca today announced positive results from a trial of a treatment for Covid-19 symptoms. New vaccine efficacy results are reported now in The Lancet. Just because Covid-19 is not considered a HCID does not mean it is not of importance.
AstraZeneca announced Friday positive results from trial data of a treatment for COVID-19 symptoms raising hopes of solutions beyond vaccines particularly for those who respond poorly to. Vaccine cocktail or heterogenous boosting strategy studies News18 was the muse of Sputnik V. Drug firm AstraZeneca on Friday announced positive results from a trial of a treatment for COVID-19 symptoms.
To ensure you have the latest information or to find out more about the trial please visit theOxford COVID-19 vaccine web hubor visit theCOVID-19 trial website. The AstraZeneca vaccine was developed in cooperation with Oxford College and is 6309 pc efficient towards the SARS-CoV-2 an infection. A late-stage trial of long-lasting AstraZeneca antibodies showed strong results in treating those who cannot take Covid-19 vaccines or are at greater risk of contracting the virus for various reasons.
To put it into context there are currently only 18 infectious diseases or strains of infectious diseases on the HCID list. Posted at Aug 20 2021 0725 PM Drug firm AstraZeneca on Friday announced positive results from a trial of a treatment for Covid-19 symptoms. The Covid-19 drug of AstraZeneca has showed positive results during its clinical trial of treatment for the coronavirus symptoms.
One of them Ebola Virus Disease has a case fatality rate of 25-90. And the results are preliminary but positive. Investigators of four randomised controlled trials conducted in the UK South Africa and Brazil report pooled results of an interim analysis of safety and efficacy against COVID-19 of the OxfordAstraZeneca chimpanzee adenovirus vectored vaccine ChAdOx1 nCoV-19 AZD1222 in adults aged 18 years and older.
How could it be other wise. The first vaccine was given to 96-year-old Jos Hermans at.
CNNs other Covid-19 trackers.
Which country will have covid 19 vaccine first. In our dataset and charts on COVID-19 vaccinations we report vaccinations performed in Israel and Palestine separately. 12 becoming one of the first Canadian. The vaccination data is needed to understand how the pandemic is evolving.
With Greece showing a total of 521 per cent of its population having received at least one dose. No a bit more equal than that. Animal drug ivermectin is not approved for use in the treatment of COVID-19.
But there has been an effort to distribute the vaccine to poorer. One recent study modeled how covid-19 is likely to spread in six countriesthe US India Spain Zimbabwe Brazil and Belgiumand concluded that if. The OxfordAstraZeneca COVID-19 vaccine sold under the brand names Vaxzevria and Covishield is a viral vector vaccine produced by the British University of Oxford British-Swedish company AstraZeneca and the Coalition for Epidemic Preparedness Innovations.
China became the first country on June 5 to approve Covid-19 vaccination for children as young as three years old. Citing a study the government claimed that the antibodies created by the indigenously made vaccine are no worse than those created by AstraZenecas vaccine. Tajikistan Becomes First Country To Make Vaccines Mandatory According to the decision of the commission vaccination against coronavirus is mandatory for citizens over 18 years of ageTajikistans ministry stated.
The global option was the first choice for more than 50 percent of participants in most countries including Germany Argentina and Brazil. British Columbia is mandating COVID-19 vaccines for all staff working in long-term care homes and assisted living facilities officials announced on Aug. To avoid another wave of Covid-19 many countries around the world are.
For this it is key to bring together the vaccination data with data on COVID-19 cases and COVID-19 deaths. Kazakhstan will introduce mandatory Covid-19 vaccinations or weekly testing for people working in groups of more than 20 the health ministry said on 23 June. In December 2020 the first dose of a fully tested vaccine manufactured by PfizerBioNTech was.
People in Belgium received the first COVID-19 vaccines developed by BioNTechPfizer on December 28. In Finland Brazil and Portugal over 40. A vaccination center in Brussels Belgium A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 COVID19.
Lebanon is to limit entry to restaurants cafes pubs and beaches to people holding vaccine certificates or those who have taken antibodies tests the tourism ministry said on 30 July. On 2 December 2020 the United Kingdoms Medicines and Healthcare products Regulatory Agency MHRA gave temporary regulatory approval for the PfizerBioNTech vaccine becoming the first country to approve this vaccine and the first country in the Western world to. With the news that Pfizers COVID-19 vaccine can prevent more than 90 of people getting the disease the race is on for countries to buy up as many doses as possible.
Denmark and Norway suspended the use of the OxfordAstraZeneca vaccine due to a small number of reports of a rare blood clot disorder. View vaccinations by country. Health Minister Mansukh Mandaviya on Tuesday said several Indian companies are increasing their production of Covid-19 vaccines and that the country may become the first.
Obviously the countries that invented dev eloped and manufacture the vaccine have first dibs for their own populations. Cyprus comes next with 558 per cent of its population having received at least a dose. The nonprofit Gavi vaccine alliance which is based in Geneva and buys vaccines for poor countries is raising 2 billion to make its own pre-purchase agreements for covid-19 immunizations.
The UK was among the first nations to roll out a comprehensive vaccination program early in 2021. The US follows with 555 per cent.
US preparing for 1-year COVID-19 booster shots. However these studies are ongoing and we now know that immunity actually lasts longer than we anticipated.

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
So how long does coronavirus vaccine immunity.

How long will covid 19 vaccine last. How long do antibodies against covid-19 stay in the body. It is not yet known how long the protection of the COVID-19 vaccine will last. Studies of two of the most prominent COVID-19 vaccines suggest they remain effective for at least six months.
Pfizer chief sees need. Studies show that COVID-19 vaccines are safe and effective. The CEO of one vaccine maker said immunity may start to fade within a year.
We will know more through ongoing research. Regardless as of now it seems like the Covid-19 vaccines should protect you for at least six months. The Johnson Johnson Moderna and Pfizer-BioNTech vaccines will likely protect against current variants of COVID-19.
Guido Vanham GV. Since people whove been vaccinated mount an even better immune response Wherry says he thinks immunity from the COVID-19 vaccines will likely last several years if not longer. Johnson Johnson meanwhile has yet to report six-month data for its single-shot vaccine.
Many hospitals have been forced to re-open coronavirus wards. That same month Pfizer reported that its vaccine was still highly effective at six months. All that will happen as quickly as possible.
We have done that with smallpox but thats the only example - and that has taken many years. Trial data for the Pfizer-BioNTech vaccine has revealed that even after 85 days of the second vaccination dose the body still had the necessary antibodies to protect against SARS CoV-2 Covaxin has claimed its COVID-19 vaccine is capable of producing antibodies which can persist in the body for six to twelve months Oxford-AstraZenecas adenovirus vaccine being manufactured at. Aug 22 2021 - Health Digest.
Data indicate that neutralising antibodies last for several months in patients with covid-19 but gently fall in number over time. A second reason why booster shots may be. In April a report in The New England Journal of Medicine NEJM said that in all 33 participants who had received the Moderna vaccine during the Phase I trial protection remained high for six months after the second shot.
That seems like a long time but last week an FDA official told CNN that the decision is likely to come within 2 months. Initially we knew immunity lasted at least six months after vaccination because the first trials had six months of data at that time. A 2004 study of individuals who were vaccinated against measles as children found strong T-cell immunity more than 30 years later.
COVID-19 vaccines have been used under the most intensive safety. Then well go to the CDC. How Long Does The COVID-19 Booster Shot Last.
She said it is not clear how soon those under the age of 12 will be approved to take the COVID-19 vaccine by the FDA. Most Israelis thought the worst of COVID-19 was behind them but infection rates have doubled in the past two weeks. We dont know how long T-cell immunity persists after COVID-19 vaccination or infection but theres evidence that T cells last for quite a while.
Children 12 years and older are now eligible to get vaccinated against COVID-19. Experts dont yet know how long a booster shot will last but are recommending to get them eight months after your final previous vaccination shot. John Greene chair of the Infectious Diseases Program at.
What do we know about how long immunity lasts after vaccination for COVID-19. What we do know is that evidence shows the Pfizer and the AstraZeneca COVID-19 vaccines prevent severe disease. However they will most likely have to be administered annually.
These side effects may affect their ability to do daily activities but they should go away in a few days. Its going to be somewhere in the. Vaccination is the best way to protect you and your loved ones from the COVID-19 virus.
It will probably never end in the sense that this virus is clearly here to stay unless we eradicate it. Theyve been working double overtime on all of these issues to review the data and then make the appropriate regulatory decisions she said. While the current COVID-19 vaccines will likely last for at least about a year they probably will not offer lifelong protection as with measles shots said Dr Kathleen Neuzil a vaccine expert at the University of Maryland.
One of the most pressing questions about COVID-19 vaccines is how long they can provide protection. Experts dont yet know how long a booster shot will last but are recommending to get them eight months after your final. Like adults children may have some side effects after COVID-19 vaccination.
Yes vaccinated people can still contract the virus especially the delta variant but the symptoms will be milder and infection is much less likely to require a trip to the hospital said Dr. The company however has released promising laboratory data showing a strong immune system response up to eight months later. And the only way to eradicate such a virus would be with a very effective vaccine that is delivered to every human being.
Ongoing trial shows Pfizer Covid-19 vaccine remains highly effective after six months Doctors are worried that coronavirus may end up being like influenza which requires a. The Moderna and Pfizer-BioNTech vaccines offer immunity against COVID-19 for at least six months and might offer protection for up to two to three years. A fourth wave is raging in Israel fast approaching the difficult days of last January.
Clinical trials are currently happening to find out if we will need booster doses on an annual or longer basis. And a real-world study from South Africa showed good protection against delta.
