COVID arm has been reported as a delayed reaction to the vaccine with a red itchy swollen or painful rash developing around the injection site. Enlarged lymph nodes.

Covid Vaccine Rare Side Effect Sees Itchy Red Rash Form After Jab Edinburgh Live
Rash after Covid vaccine.
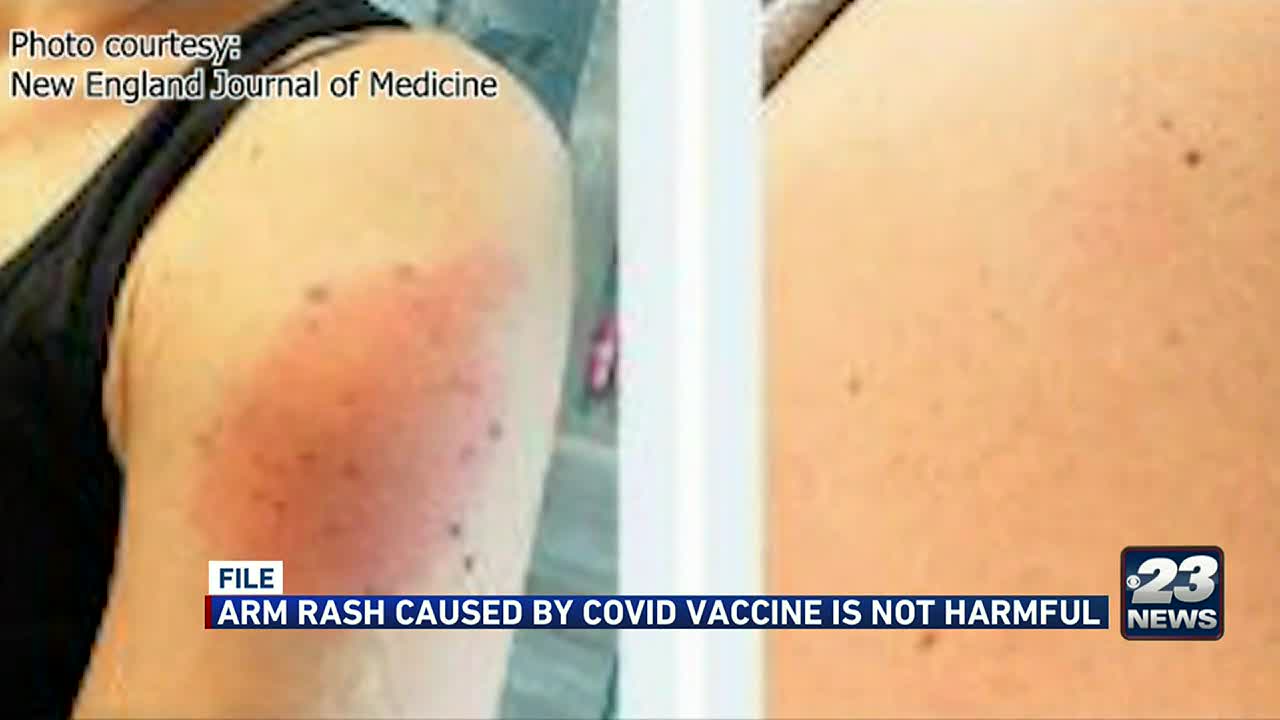
Red rash on arm after astrazeneca covid vaccine. Pain swelling tenderness redness or itching at the injection site tiredness headache muscle pain nausea fever and chills feeling unwell joint pain. Freeman said COVID arm can be itchy red and swollen. WEDNESDAY May 12 2021 HealthDay News -- In rare cases people who receive the two-dose Moderna COVID-19 vaccine may experience a red itchy patch of skin a few days later at the injection.
According to the Center for Disease Control and Prevention CDC website Covid arm rash can be large and red itchy swollen or painful. These rashes typically clear up within three to four days according to the study. Common side effects after COVID-19 Vaccine AstraZeneca include.
Leigh King of Wishaw in North Lanarkshire got her first dose of the Oxford-AstraZeneca vaccine on March 12 but is still suffering from intense pain. Data from the ZOE Covid Symptom Tracking Study a surveillance tool that tracks Britains outbreak showed skin burning rashes and red welts on the lips and face are possible side effects from. Presenting anywhere from five to 10 days after receiving the vaccine the extremely rare COVID arm phenomenon may leave people with itchy and.
A rash that looks like small bruises or bleeding under the skin. The rash is likely a delayed allergic reaction. I think sometimes this.
COVID arm is a term coined by experts to describe a delayed itchy rash or dull pain at the injection site after youve received a COVID-19 vaccine. Having a sore arm after you get a vaccine is pretty common but some people who get their first dose of a COVID vaccine have ended up with a red or itchy rash on their arm. Immune thrombocytopenic purpura ITP causes red spots under the skin that look like a rash.
Any vaccine can cause pain redness or swelling at the injection site a day or two after you get the shot including the Moderna and Pfizer COVID-19 vaccines. It usually presents as a big red splotch on your arm. Leigh King from North Lanarkshire in Scotland claimed her skin.
Most reactions like hives swelling and wheezing occur within 4 hours of the vaccine says the CDC. Some people may experience a rash on their injection arm after getting the Moderna COVID-19 vaccine. Covid arm is a delayed reaction and can take up to a week to appear after the jab.
Mild side effects from the jab may include tiredness or aches Image. One possible side effect they have noticed is people reporting a bright red and potentially swollen or tender patch on their arm which can develop days after the vaccine. As with any vaccine you may have some side effects after receiving a COVID-19 vaccine.
The CDC says some amount of arm pain or redness is an expected side effect of any vaccine including the COVID vaccine but some patients are reporting symptoms lasting for over a week after getting their injection. RED ALERT Get medical help if you spot these symptoms after AstraZeneca Covid vaccine experts say. It occurs due to the immune system in your skin over-reacting.
Currently the trend is clearly linked to Moderna. Less common side effects after COVID-19 Vaccine AstraZeneca include. Mothers face arm and chest erupts in agonising red rash after getting AstraZenecas Covid vaccine - as 41-year-old claims she is still in unbearable pain two weeks later.
A mothers face arms chest back and legs erupted into an agonising red rash after getting the AstraZeneca coronavirus vaccine. This is the leg of someone with ITP who had the condition after Covid.
The Johnson Johnson product is an adenovirus vaccine or a viral vector vaccine. The Johnson Johnson vaccine delivers DNA to your cells to make the spike protein.

Coronavirus Eu Rejects Some Johnson Johnson Covid Vaccines Over Contamination News Dw 11 06 2021
Johnson Johnsons Janssen JJJanssen COVID-19 Vaccine.
Is johnson and johnson working on a coronavirus vaccine. It teaches your immune system to recognize and destroy the coronavirus. January 2020 Johnson Johnson begins work on a coronavirus vaccine. The Johnson and Johnson COVID-19 vaccine is the third COVID-19 vaccine that the Food and Drug Administration FDA authorized for emergency use.
The three COVID-19 vaccines cause similar side effects including mild flu-like symptoms and injection-site reactions. Johnson Johnson on Wednesday became the latest drugmaker to begin work on developing a vaccine for a new coronavirus that has already killed more than 100 people in China as health authorities. Johnson Johnson is currently working on a booster to help its Covid-19 vaccine deal with coronavirus variants CEO Alex Gorsky told CNNs Jim Sciutto on Monday.
Different vaccines work in slightly different ways. Johnson Johnsons NYSEJNJ Covid-19 vaccine is one of the most closely watched shots against the novel coronavirus given that it is backed by one of the worlds largest pharma companies and. March Johnson Johnson receives 456 million from the United States government to help develop and produce the vaccine.
Department of Health and Human Services and Johnson. A single dose of the Covid-19 vaccine made by Johnson Johnson is highly effective in preventing severe illness and death from the Delta and Beta variants of the coronavirus data from a. The significant expansion of the existing partnership between the Janssen Pharmaceutical Companies of Johnson.
CNN The Johnson Johnson coronavirus vaccine provides immunity that lasts at least eight months and it appears to provide adequate protection against the. Johnson. The Johnson Johnson shot uses a weakened common cold virus called an adenovirus as a vehicle to deliver a single coronavirus gene into human cells.
Severe reactions to the vaccines are very rar e. A study on vaccine efficacy against COVID-19 variants has shown that the Johnson Johnson vaccine works better against the Delta variant and gets better over time with both Delta and Beta variants. 2 The company worked with its pharmaceutical arm Janssen to develop the vaccine under the project names Ensemble and Ensemble 2.
This follows a study conducted where 10 participants received one dose and 10 participants received two doses of Johnson Johnson COVID-19 vaccine up to eight months. Monica Gandhi an infectious disease specialist with the University of California San Francisco said there is currently no data suggesting we need to boost the Johnson Johnson vaccine. The Johnson Johnson COVID-19 vaccine Theres little data that shows how effective the Johnson Johnson JJ single-shot COVID-19 vaccine is at protecting against the Delta variant.
Here are six things we know about the Johnson Johnson vaccine. Reuters - Johnson Johnson on Wednesday became the latest drugmaker to begin work on developing a vaccine for a new coronavirus that has already killed more than 100 people in China as health authorities race to contain the outbreak. There also isnt any strong evidence that we are seeing more breakthrough infections in people who got the JJ shot versus an mRNA vaccine like Pfizer or Moderna.
The Centers for Disease Control and Prevention CDC and the US Food and Drug Administration FDA recommended that use of JJJanssen COVID-19 Vaccine resume in the United States effective April 23 2021. On February 27 Johnson Johnsons vaccine against the coronavirus disease 2019 COVID-19 became the third COVID-19 vaccine to receive emergency use authorization EUA from the Food and Drug Administration FDA. An adenovirus acts as a delivery vehicle carrying coronavirus genetic material DNA.
Here is how it works. There are currently three authorized COVID-19 vaccines available in the US. Two mRNA vaccines Pfizer and Moderna and one viral vector vaccine Johnson Johnson.
Current data for the eight months studied so far show that the single-shot Johnson Johnson COVID-19 vaccine generates a strong neutralizing antibody. The Trump administration is working with Johnson Johnson Pharmaceuticals to create a vaccine for the coronavirus. Announcing data from two Phase 12a studies Johnson Johnson JNJ said an additional dose of the companys single-dose COVID-19 vaccine generated a nine-fold rise in.
One of them Ebola Virus Disease has a case fatality rate of 25-90. As of November 25 2020 among participants who had received at least 1 dose of vaccine or placebo vaccine15185 placebo15166 unsolicited adverse events that occurred within 28 days following each vaccination were reported by 239 of participants n3632 who received Moderna COVID19 Vaccine and 216 of participants n3277 who received placebo.
Debunked No Covid 19 Vaccines Didn T Skip Or Fail Animal Trials
The rate of deaths occurring among vaccinated individuals isnt higher than in unvaccinated people indicating that the vaccines.

Covid vaccine animal trial results. Immunization of non-human primates rhesus macaques with BNT162b2 a nucleoside-modified messenger RNA modRNA candidate that expresses the SARS-CoV-2 spike glycoprotein resulted in strong. All three COVID-19 vaccines authorized for emergency use by the US. This article was produced by the Reuters Fact Check team.
Chris Magee head of policy and media at UK non-profit Understanding Animal Research UAR told Full Fact that in the case of Covid-19 vaccines data already existed to indicate the vaccines were safe which enabled researchers to run animal trials alongside the early stages of human trials. As a result Covid-19 was removed from the list of HCIDs. In each case trial results.
Posts claiming that COVID-19 vaccine producers skipped animal trials due to the animals in those trials dying are false. To put it into context there are currently only 18 infectious diseases or strains of infectious diseases on the HCID list. The virus multiplied unchallenged and all animals involved in the experiment died from various causes.
In all the monkeys who got the high doses of the. Significant reductions of SARS-CoV two days after challenge was seen for all vaccines and prior live SARS-CoV. He also said animals.
Results All vaccines induced serum neutralizing antibody with increasing dosages andor alum significantly increasing responses. Reports of deaths occurring after COVID-19 vaccination alone arent evidence that the vaccines were the cause of deaths. These doctors have become concerned about COVID-19 mRNA vaccine safety.
COVID-19 vaccines authorized for use in the US. At the highest dose studied no virus was recovered from vaccinated monkeys from the throat lung or. The results if they hold up in humans suggest that the vaccine may be able to protect against Covid-19 in both the upper and lower airways.
Both companies recognized that mRNA vaccines work very differently in animals compared to humans. Animal Trials Cut Short. Pfizer Moderna and Johnson Johnson have all said in press releases that they tested their COVID-19 vaccines on animals in pre-clinical trials.
The ferrets showed no adverse reaction and seemed to be completely healthy. Pfizer and BioNTech Announce Data from Preclinical Studies of mRNA-based Vaccine Candidate Against COVID-19. In animal studies after mRNA injections have been administered to cats when the virus arrived once again into the body it arrived like a Trojan Horse undetected by the cats own immune system.
More than 700 human trials of potential HIVAIDS vaccines have been conducted all of which gave encouraging results in animals including monkeys and chimpanzees yet not one has worked in humans. In 2005 an animal study was done with SARS-CoV-1 mRNA vaccines on ferrets. This observation is in marked contrast to the results reported from Sinovac trial.
In fact some HIV vaccines actually increase the risk of HIV in humans. There is convincing evidence that most epidemics and pandemics including Covid-19 have actually been caused by humans playing god and exploiting animals. Along with the vaccines for Pfizer and Moderna both passing animal trials they also passed clinical trials.
On day 58 challenged mice were sacrificed and lungs obtained for virus and histopathology. Posts online appeared to suggest that the animal trial phase was skipped completely when testing the two vaccines. Then on November 17th Reuters wrote in a cheerleading piece for Pfizer and Moderna How two companies sprinted ahead in extraordinary race for a COVID vaccine The article said.
And UK are not experimental and have all completed animal and clinical trials. Wednesday September 09 2020 - 0745am. Housed in fat cells.
Had the animals died during this process he said the human trials would have been immediately halted. This new safety information involves the Pfizer mRNA-based vaccine known as BNT162b2 or Comirnaty The FOIA documents reveal animal study results demonstrating that the Pfizer mRNA-based vaccine does not remain at the injection site but rather appears to spread widely after injection. After some time the ferrets were exposed to the SARS-CoV-1 coronavirus in the wild.
The fact that they were not indicates the animals did not die unexpectedly. And as models of human diseases NHPs have failed for Alzheimers and Parkinsons diseases. Just because Covid-19 is not considered a HCID does not mean it is not of importance.
University of Pennsylvania professor of medicine Dr. Two doses were administered in the same manner as the SARS-CoV-2 aka COVID-19 mRNA Vaccines. Food and Drug Administration were tested in animals and in clinical trials on humans before receiving authorization.
A clinical trial for an experimental coronavirus vaccine has begun recruiting participants in Seattle but researchers did not first show that the vaccine triggered an immune response in animals. Drew Weissman who has been studying mRNA and mRNA vaccines for decades said they do not cause dangerous inflammation to animals.
If you have a history of immediate onset anaphylaxis to multiple classes of drugs or unexplained anaphylaxis please also refer to the additional information at. Experts say that depends on the type of reaction you had.

A Guide To Who Can Safely Get The Pfizer Biontech Covid 19 Vaccine
However Winokur said people who have had.

Can i have the covid vaccine if i have allergies to antibiotics. Table 2 summaries their opinions. Note that most support vaccination in those with prior allergies and some even those that have had prior anaphylaxis with close observation. Centers for Disease Control and Prevention has reported that allergic reactions to the COVID-19.
Its recommended that if youve had an allergic reaction any of the ingredients in the vaccine you should not get it. If you had an allergic reaction to the first dose of a COVID-19 vaccine the CDC doesnt recommend getting the second dose. And this needs to be done by a prescription of a healthcare provider.
And to only be receiving antibiotics if they are significantly ill where the healthcare provider is suspicious of on top of the COVID-19 the patient having a bacterial infection. International organisations or regulatory bodies have made statements on Covid-19 vaccine use and allergies. None of the currently approved UK COVID-19.
Even those who have allergies to penicillin antibiotics or types of medications are not at risk if they get a COVID-19 vaccine. AS we implement our national Covid-19 vaccination programme those of us who are healthcare professionals have numerous friends and acquaintances asking us about the safety of the Covid-19 vaccines. Previous advice from the MHRA said people with a range of allergies to food and medicines should not be given the Pfizer vaccine.
But overall having allergies doesnt exclude you from getting the vaccine. Additionally if you are taking antibiotics you can also still get vaccinated. Anaphylaxis can occur after taking a drug from a bee sting eating some food item etc and not just vaccines.
We often never know who is going to react in this way. The good doctor asked if I had any history of allergic reactions to penicillin. Also the vaccine wont have an effect on whatever bacterial infection you are taking the antibiotics for.
As the Medicines and Healthcare products Regulatory Agency MHRA has continued their close surveillance of the vaccine roll out they have now advised that individuals with a history of allergy or anaphylaxis to any food can receive any COVID-19 vaccine as long as they are not known to be allergic to any component excipient of the vaccine. Yes allergy to penicillins is not a contraindication to the PfizerBioNTech or AstraZeneca COVID-19 vaccine or Moderna vaccine. Dr June Raine said growing evidence from a pool of at least 800000 people in the UK and probably 15 million people in the US who have had the vaccine has raised no additional concerns.
If you have had a severe allergic reaction or an immediate allergic reactioneven if it was not severeto any ingredient in an mRNA COVID-19 vaccine you should not get either of the currently available mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna. But all do not support vaccination in anyone who has had a. Last year I took my first penicillin vaccine.
If You Are Allergic to an Ingredient in a COVID-19 Vaccine. In other words if you have a mild cough runny nose or diarrhea but are COVID negative you can get the vaccine. Antibiotics do not affect the vaccine and it is OK to continue them.
I cant say because I am a penicillin virgin. He continued Almost everyone else even those who have histories of allergies to other antibiotics other types of injections generally can receive these vaccines MORE.
US preparing for 1-year COVID-19 booster shots. However these studies are ongoing and we now know that immunity actually lasts longer than we anticipated.

Covid 19 Vaccinations What S The Progress Science In Depth Reporting On Science And Technology Dw 14 08 2021
So how long does coronavirus vaccine immunity.

How long will covid 19 vaccine last. How long do antibodies against covid-19 stay in the body. It is not yet known how long the protection of the COVID-19 vaccine will last. Studies of two of the most prominent COVID-19 vaccines suggest they remain effective for at least six months.
Pfizer chief sees need. Studies show that COVID-19 vaccines are safe and effective. The CEO of one vaccine maker said immunity may start to fade within a year.
We will know more through ongoing research. Regardless as of now it seems like the Covid-19 vaccines should protect you for at least six months. The Johnson Johnson Moderna and Pfizer-BioNTech vaccines will likely protect against current variants of COVID-19.
Guido Vanham GV. Since people whove been vaccinated mount an even better immune response Wherry says he thinks immunity from the COVID-19 vaccines will likely last several years if not longer. Johnson Johnson meanwhile has yet to report six-month data for its single-shot vaccine.
Many hospitals have been forced to re-open coronavirus wards. That same month Pfizer reported that its vaccine was still highly effective at six months. All that will happen as quickly as possible.
We have done that with smallpox but thats the only example - and that has taken many years. Trial data for the Pfizer-BioNTech vaccine has revealed that even after 85 days of the second vaccination dose the body still had the necessary antibodies to protect against SARS CoV-2 Covaxin has claimed its COVID-19 vaccine is capable of producing antibodies which can persist in the body for six to twelve months Oxford-AstraZenecas adenovirus vaccine being manufactured at. Aug 22 2021 - Health Digest.
Data indicate that neutralising antibodies last for several months in patients with covid-19 but gently fall in number over time. A second reason why booster shots may be. In April a report in The New England Journal of Medicine NEJM said that in all 33 participants who had received the Moderna vaccine during the Phase I trial protection remained high for six months after the second shot.
That seems like a long time but last week an FDA official told CNN that the decision is likely to come within 2 months. Initially we knew immunity lasted at least six months after vaccination because the first trials had six months of data at that time. A 2004 study of individuals who were vaccinated against measles as children found strong T-cell immunity more than 30 years later.
COVID-19 vaccines have been used under the most intensive safety. Then well go to the CDC. How Long Does The COVID-19 Booster Shot Last.
She said it is not clear how soon those under the age of 12 will be approved to take the COVID-19 vaccine by the FDA. Most Israelis thought the worst of COVID-19 was behind them but infection rates have doubled in the past two weeks. We dont know how long T-cell immunity persists after COVID-19 vaccination or infection but theres evidence that T cells last for quite a while.
Children 12 years and older are now eligible to get vaccinated against COVID-19. Experts dont yet know how long a booster shot will last but are recommending to get them eight months after your final previous vaccination shot. John Greene chair of the Infectious Diseases Program at.
What do we know about how long immunity lasts after vaccination for COVID-19. What we do know is that evidence shows the Pfizer and the AstraZeneca COVID-19 vaccines prevent severe disease. However they will most likely have to be administered annually.
These side effects may affect their ability to do daily activities but they should go away in a few days. Its going to be somewhere in the. Vaccination is the best way to protect you and your loved ones from the COVID-19 virus.
It will probably never end in the sense that this virus is clearly here to stay unless we eradicate it. Theyve been working double overtime on all of these issues to review the data and then make the appropriate regulatory decisions she said. While the current COVID-19 vaccines will likely last for at least about a year they probably will not offer lifelong protection as with measles shots said Dr Kathleen Neuzil a vaccine expert at the University of Maryland.
One of the most pressing questions about COVID-19 vaccines is how long they can provide protection. Experts dont yet know how long a booster shot will last but are recommending to get them eight months after your final. Like adults children may have some side effects after COVID-19 vaccination.
Yes vaccinated people can still contract the virus especially the delta variant but the symptoms will be milder and infection is much less likely to require a trip to the hospital said Dr. The company however has released promising laboratory data showing a strong immune system response up to eight months later. And the only way to eradicate such a virus would be with a very effective vaccine that is delivered to every human being.
Ongoing trial shows Pfizer Covid-19 vaccine remains highly effective after six months Doctors are worried that coronavirus may end up being like influenza which requires a. The Moderna and Pfizer-BioNTech vaccines offer immunity against COVID-19 for at least six months and might offer protection for up to two to three years. A fourth wave is raging in Israel fast approaching the difficult days of last January.
Clinical trials are currently happening to find out if we will need booster doses on an annual or longer basis. And a real-world study from South Africa showed good protection against delta.
